Research
Neocortical cultures unveil a tapestry of diverse human astrocytes
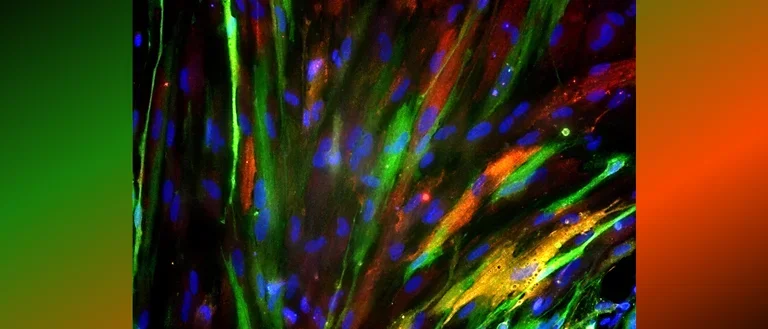
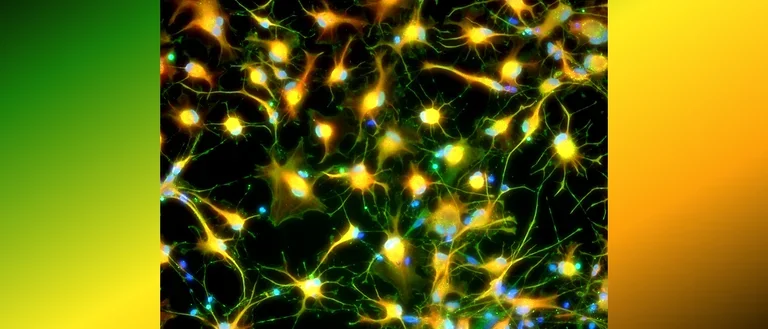
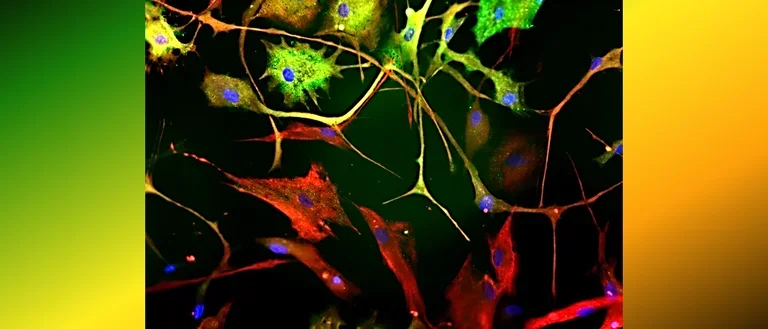
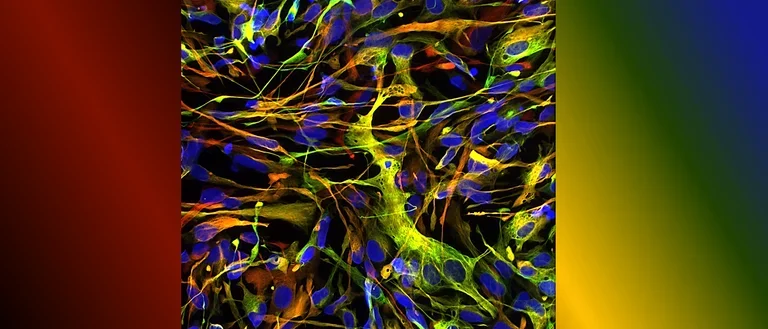
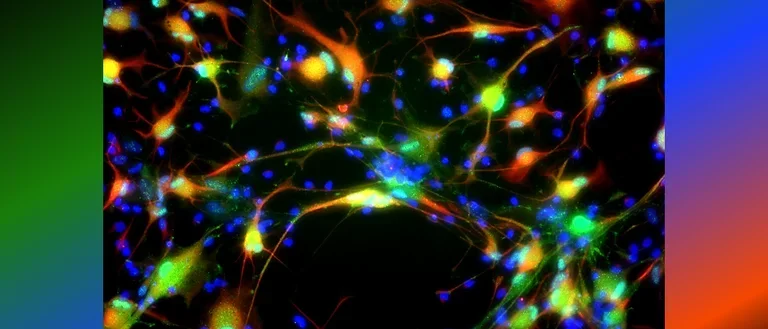
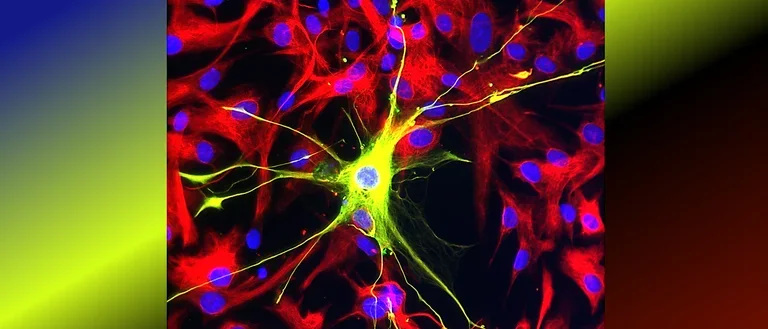
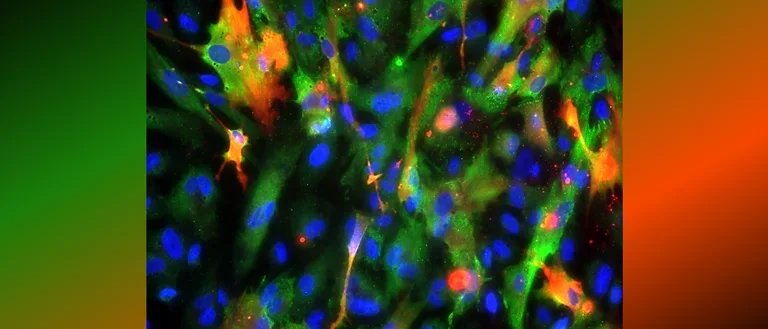
Human astrocytes come in various shapes and express different markers to perform their complex roles in the brain. This diversity is illustrated in the provided images, showing both star-shaped and fibrous astrocytes from gray and white matter. The colorful spectrum of marker expression highlights astrocyte subpopulations specific to humans in their diversity.
Fetal neocortical human astrocytes differentiated in vitro show diverse markers and display a range of morphotypes
From research findings to translations to clinical use
To accelerate research findings towards clinical use we need to bridge barriers and set in motion bidirectional communication between researchers and clinicians. We need to design research that builds parallels by using noninvasive tools like blood-based biomarkers, establish human models and harmonize our language and develop a common data structure. Then we can draw inferences to make research clinically relevant.
Industry partners
Astrocyte Pharmaceuticals - CEO: Bill Korinek, Co-founder: James Lechleiter
BrainBox Solution - CSO: Tim Van Meter, CEO: Donna Edmonds
EnCor Biotechnology Inc.: CEO and Founder: Gerry Shaw
Astrocytes govern brain activity, integrity and equilibrium
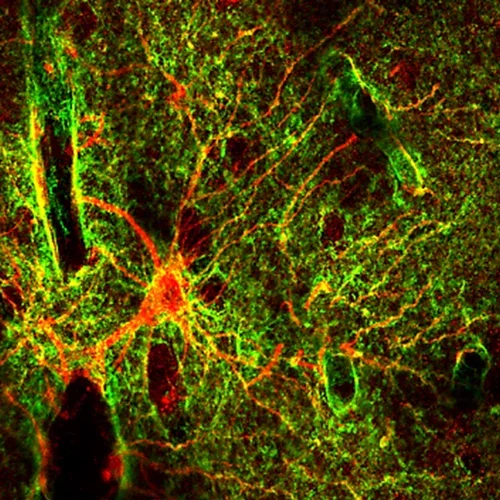
We study glial cells called astrocytes, caregivers and gatekeepers of the brain. Astrocytes supply vital nutrients in response to neural activity and restore equilibrium following injuries. Thus, damage and loss of these crucial cells hamper essential restorative processes following neurotrauma. Consequently, enhancing astrocyte viability is a promising therapeutic approach for central nervous system injuries.
A human astrocyte (red) of the gray matter neocortex is shown with its arms and elaborate fine endings, called endfeet (green) are shown wrapping around a blood vessel on the left and reaching every neural crevice in the gray matter of its territory.
From Proteomic Profiles to Biomarker Signatures of Brain Injury
Our patented "traumatome" contains the comprehensive proteome released by injured astrocytes. After trauma, including mild hits, wounded astrocytes rapidly release vesicles primarily containing metabolic enzymes and vesicle-associated proteins. This acute profile suggests wounded cells encounter metabolic deficits. More severe trauma and with progressive time cell death occurs with cytoskeletal fragmentation. Through rigorous analysis, we identified brain-specific proteins detectable in circulation, for unique cell-fate informed signatures of brain injury. In collaboration with our licensing partners, we are developing targeted blood tests for the most promising astrocyte injury-defined, AID biomarkers. Biomarkers provide a minimally invasive, rapid diagnostic tool for TBI including concussions, potentially improving triage, treatment decisions, and patient outcomes.
Biomarker signatures can reveal that status of the injured brain
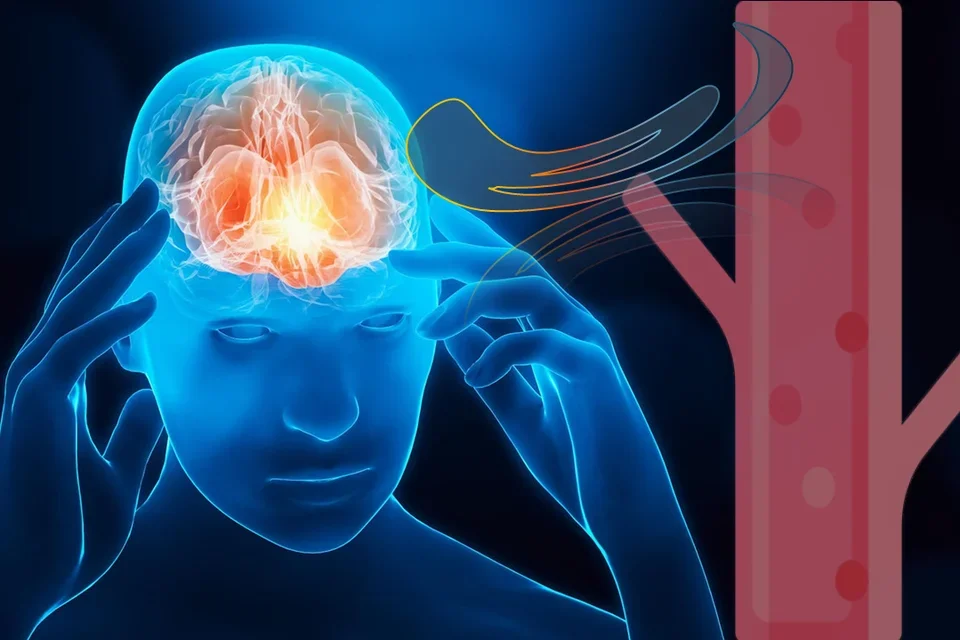
After a TBI the brain releases a specific set of proteins from wounded and dying brain cells are washed out into circulation via various pulsating fluid streams. From blood biomarker signatures we can gain insights about the injury types and stages to improve patient evaluation and care.
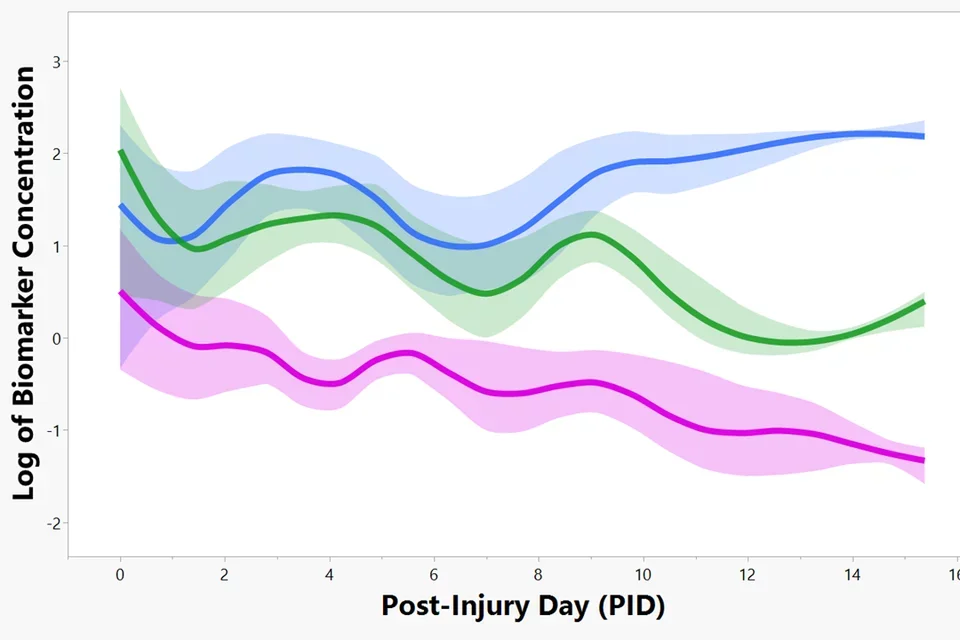
UCLA’s Ronald Reagan hospital Neuro-ICU clinical study explores AID biomarker profiles in TBI patients
Funded by the Dana Foundation “First in man” Clinical Neuroscience Award, our clinical study demonstrates Astrocyte Injury-Defined (AID) biomarkers tracking TBI progression over two weeks in 24 TBI patients from UCLA’s Neuro-Intensive Care Unit, led by Dr. Paul Vespa. These biomarkers peak on injury day with each showing unique secondary peaks in cerebrospinal fluid (CSF) that correlate with adverse events (Brain lipid binding protein, BLBP, pink; Aldolase C, ALDOC, blue; Glial fibrillary acidic protein and fragments, GFAP, green).
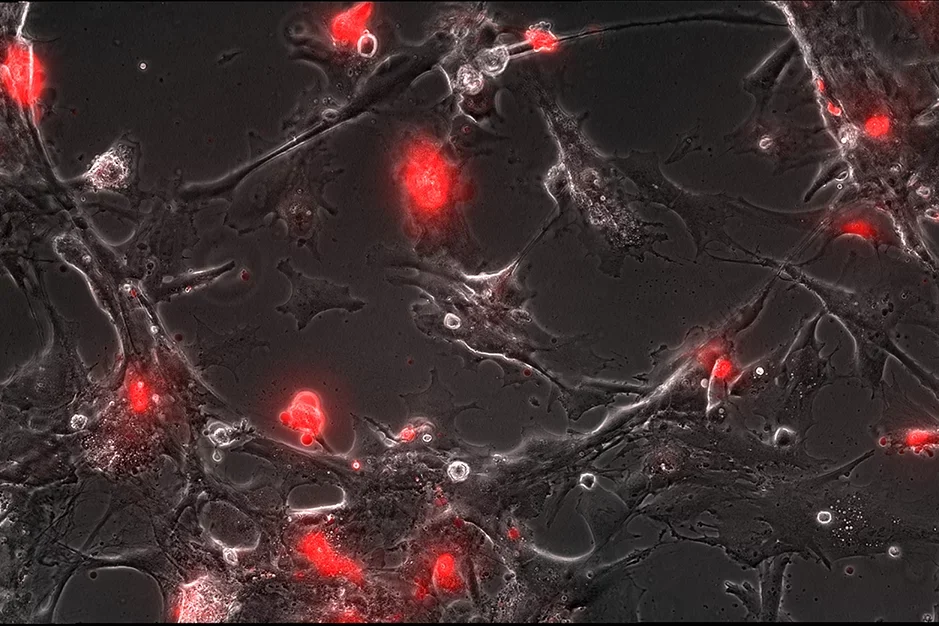
Astrocyte wounding after trauma is selective
Only a subset of stretched astrocytes remain injured, flooded by the red dye, while other cells rapidly reseal and transform into reactive astrocytes, revealed by timelapse imaging.
Hallmark of neurodegeneration in human trauma culture model mirrors postmortem tissue pathology
Wounded human astrocytes have leaky membranes (flooded by the red dye) and display swollen, beaded or entirely amputated processes (GFAP, green). These pathological features are known as clasmatodendrosis in postmortem brains with a history of trauma and evident neurodegeneration.
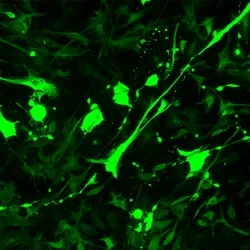
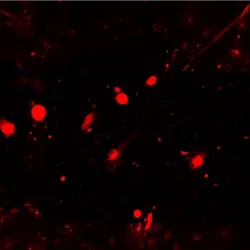
Wounded astrocytes eject vesicular biomarkers
Wounded astrocytes (red) show membrane blebbing and vesicle budding that is dynamic and can end in cell “explosion” as seen in timelapse. The unfolding cell trauma is associated with ALDOC biomarker -filled vesicle release (green).
Cells to solutions: Human trauma model reveals drug’s neuroprotective fingerprint
Neural progenitors are scaled up and then matured on flexible membranes. Abrupt pressure-pulses stretch cultures shearing and wounding cells. A promising energy-stimulating compound developed by Astrocyte Pharmaceuticals and Dr. James Lechleiter, UTH, is applied applied to traumatized cultures for testing human dose-response kinetic. Therapeutic efficacy is assayed using large field of view live imaging and unbiased automated image analysis as well as measuring biomarkers in surrounding fluids acting as pharmacodynamic monitors.
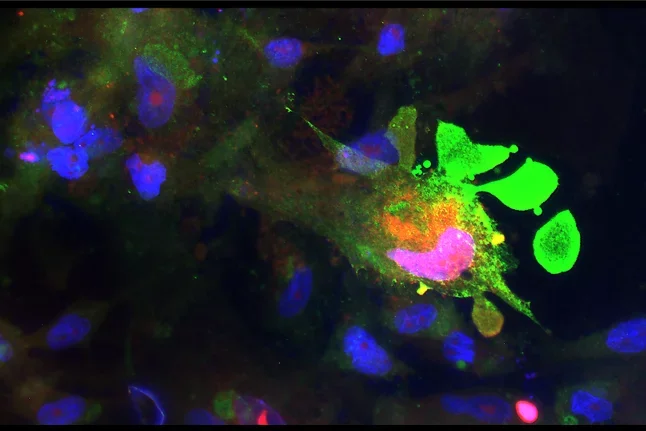
Glial scar formation
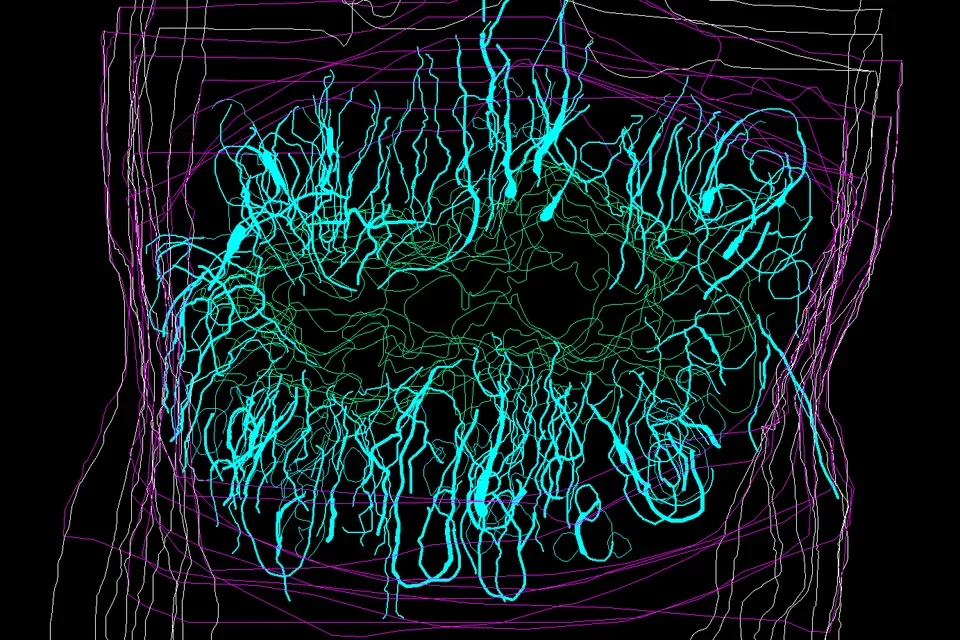
After spinal cord injury astrocytes (tiel) assembly to form the glial scar, a neuroprotective boundary around the injury site preventing invasion of non-neural cells. Nine days after spinal crush in the mouse these elongated astrocyte are here shown in their regeneration permissive “open configuration”.